Summary:Chapter 9 of Class 12 Chemistry focuses on Coordination Compounds, which are complex chemical substances formed by the coordination of metal ions with molecules or ions called ligands. These compounds have a central metal atom or ion surrounded by ligands, which donate electron pairs to the metal, forming coordinate covalent bonds.
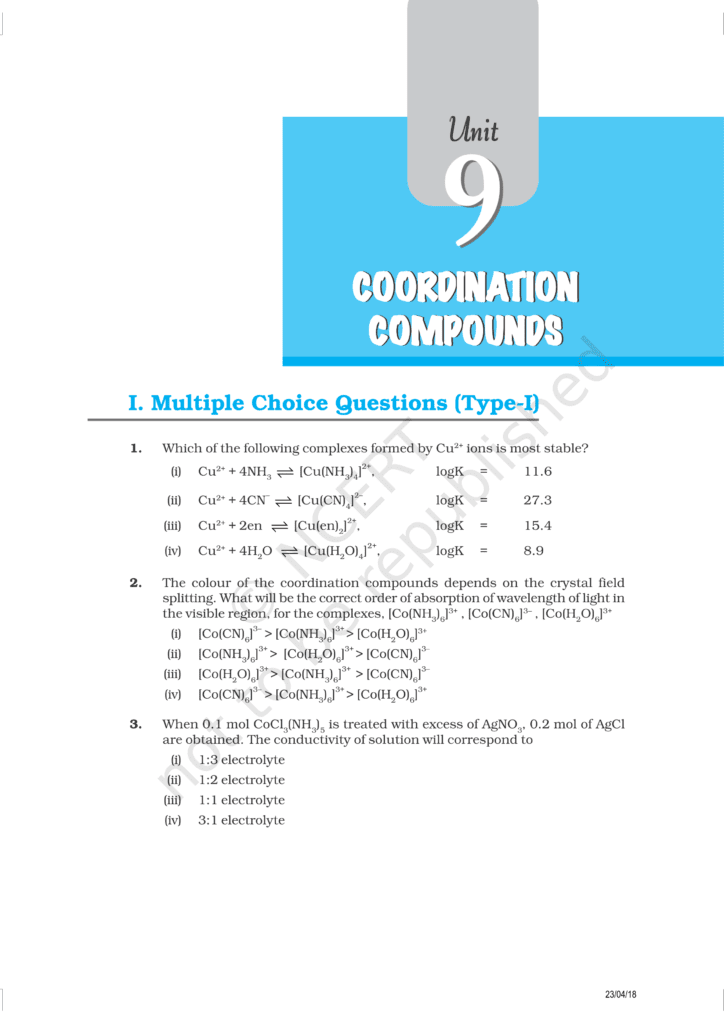
Class 12 Chemistry Chapter 9 Coordination Compound Book 📚 download PDF files 👇👇
The chapter begins by explaining the basic concepts of coordination compounds, including the definitions of ligands, coordination numbers, and coordination spheres. It discusses various types of ligands such as monodentate, bidentate, and polydentate, and introduces the concept of chelation, where ligands form rings by attaching to the metal ion at multiple points.
The chapter also covers the nomenclature of coordination compounds, providing systematic rules for naming these compounds based on IUPAC guidelines. It further explores the structural and stereoisomerism found in coordination compounds, explaining geometrical and optical isomerism in detail.
An important part of the chapter is the Valence Bond Theory (VBT) and Crystal Field Theory (CFT), which explain the bonding, color, and magnetic properties of coordination compounds. The VBT explains the formation of hybrid orbitals in the metal ion, while the CFT describes the splitting of d-orbitals in the presence of ligands and how this affects the properties of the complex.
The chapter concludes with the applications of coordination compounds in various fields, including medicine, metallurgy, and industrial processes. The study of coordination compounds is essential for understanding the complex chemistry of metals and their interactions with other molecules.
Questions with Answers
1. What is a coordination compound?
A coordination compound is a chemical substance in which a central metal atom or ion is bonded to a set of surrounding ligands through coordinate covalent bonds.
2. Define ligands and give examples.
Ligands are ions or molecules that donate electron pairs to the central metal atom or ion to form a coordination compound. Examples include H₂O, NH₃, Cl⁻, and CN⁻.
3. What is coordination number?
Coordination number is the number of ligand donor atoms to which the central metal atom or ion is directly bonded in a coordination compound.
4. Explain the term ‘chelation’.
Chelation is the formation of a coordination compound where a ligand forms multiple bonds with the central metal ion, creating a ring structure. EDTA is an example of a chelating agent.
5. What is the difference between a monodentate and a polydentate ligand?
A monodentate ligand binds to the central metal atom or ion through a single donor atom, while a polydentate ligand can bind through multiple donor atoms.
6. How are coordination compounds named according to IUPAC nomenclature?
The name of a coordination compound begins with the ligands in alphabetical order, followed by the central metal atom/ion. The oxidation state of the metal is indicated in Roman numerals in parentheses. For example, [Co(NH₃)₆]Cl₃ is named hexamminecobalt(III) chloride.
7. What is geometrical isomerism in coordination compounds?
Geometrical isomerism occurs when ligands occupy different positions around the central metal atom, leading to different spatial arrangements. This is commonly seen in square planar and octahedral complexes.
8. Describe optical isomerism in coordination compounds.
Optical isomerism occurs when coordination compounds can exist as non-superimposable mirror images, known as enantiomers. These isomers rotate plane-polarized light in opposite directions.
9. What is the Valence Bond Theory (VBT) as applied to coordination compounds?
VBT explains the bonding in coordination compounds by assuming that the metal ion forms hybrid orbitals, which then overlap with the ligand orbitals to form coordinate covalent bonds.
10. Explain the Crystal Field Theory (CFT).
CFT describes the interaction between the central metal ion’s d-orbitals and the surrounding ligands, leading to the splitting of d-orbitals into different energy levels. The arrangement of ligands around the metal ion determines the extent of splitting.
11. Why do coordination compounds show different colors?
Coordination compounds show different colors due to the splitting of d-orbitals in the metal ion when ligands are present. The absorption of light corresponding to the energy difference between split d-orbitals leads to the appearance of color.
12. What is the role of ligands in the stability of coordination compounds?
Ligands stabilize coordination compounds by donating electron pairs to the central metal ion, forming strong coordinate bonds. Chelating ligands, in particular, increase the stability of the complex.
13. How do coordination compounds exhibit magnetic properties?
The magnetic properties of coordination compounds depend on the presence of unpaired electrons in the metal ion’s d-orbitals. Compounds with unpaired electrons are paramagnetic, while those with all paired electrons are diamagnetic.
14. What is a coordination sphere?
The coordination sphere consists of the central metal ion and its attached ligands, enclosed in square brackets. The ions or molecules outside the brackets are called counter ions.
15. Explain the term ‘ambidentate ligand’.
An ambidentate ligand can bind to the central metal ion through two different atoms. For example, the SCN⁻ ion can bind through either the sulfur or nitrogen atom.
16. What is the use of EDTA in coordination chemistry?
EDTA (ethylenediaminetetraacetic acid) is a polydentate ligand used as a chelating agent to bind metal ions in various applications, including water softening and as a treatment for heavy metal poisoning.
17. How do coordination compounds play a role in biological systems?
Coordination compounds are crucial in biological systems, such as in hemoglobin, where iron is coordinated to oxygen, enabling the transport of oxygen in the blood.
18. What is meant by the effective atomic number (EAN)?
The effective atomic number is the total number of electrons surrounding the central metal atom in a coordination compound, including both its own electrons and those donated by the ligands.
19. Describe the application of coordination compounds in medicine.
Coordination compounds are used in medicine, for example, in chemotherapy, where cisplatin, a platinum-based coordination compound, is used to treat cancer.
20. Why is the study of coordination compounds important in analytical chemistry?
Coordination compounds are important in analytical chemistry for complexometric titrations, where they are used to determine the concentration of metal ions in solutions.
21. Explain linkage isomerism in coordination compounds.
Linkage isomerism occurs when a ligand can coordinate to the metal ion through different atoms, resulting in different isomers. For example, [Co(NO₂)(NH₃)₅]Cl₂ and [Co(ONO)(NH₃)₅]Cl₂ are linkage isomers.
22. What are the uses of coordination compounds in catalysis?
Coordination compounds act as catalysts in various industrial processes, such as in the hydrogenation of alkenes using Wilkinson’s catalyst, a rhodium-based coordination compound.
23. What is the significance of the chelate effect?
The chelate effect refers to the enhanced stability of coordination compounds formed by chelating ligands compared to those formed by monodentate ligands. This effect is important in biological and industrial processes.
24. Explain the concept of crystal field splitting energy (Δ)
Crystal field splitting energy (Δ) is the energy difference between the split d-orbitals in a coordination compound. The magnitude of Δ determines the compound’s color and magnetic properties.
25. What is a homoleptic and a heteroleptic complex?
A homoleptic complex is a coordination compound in which all ligands are identical, while a heteroleptic complex contains different types of ligands.

Get involved!
Comments